Bimolecular Fluorescence Complementation (BiFC) Assay Service
BiFC Service Overview
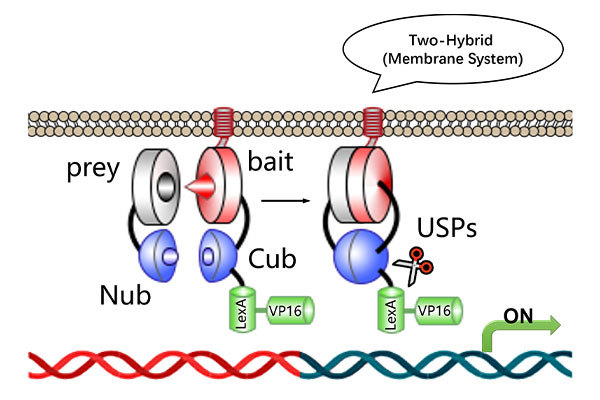
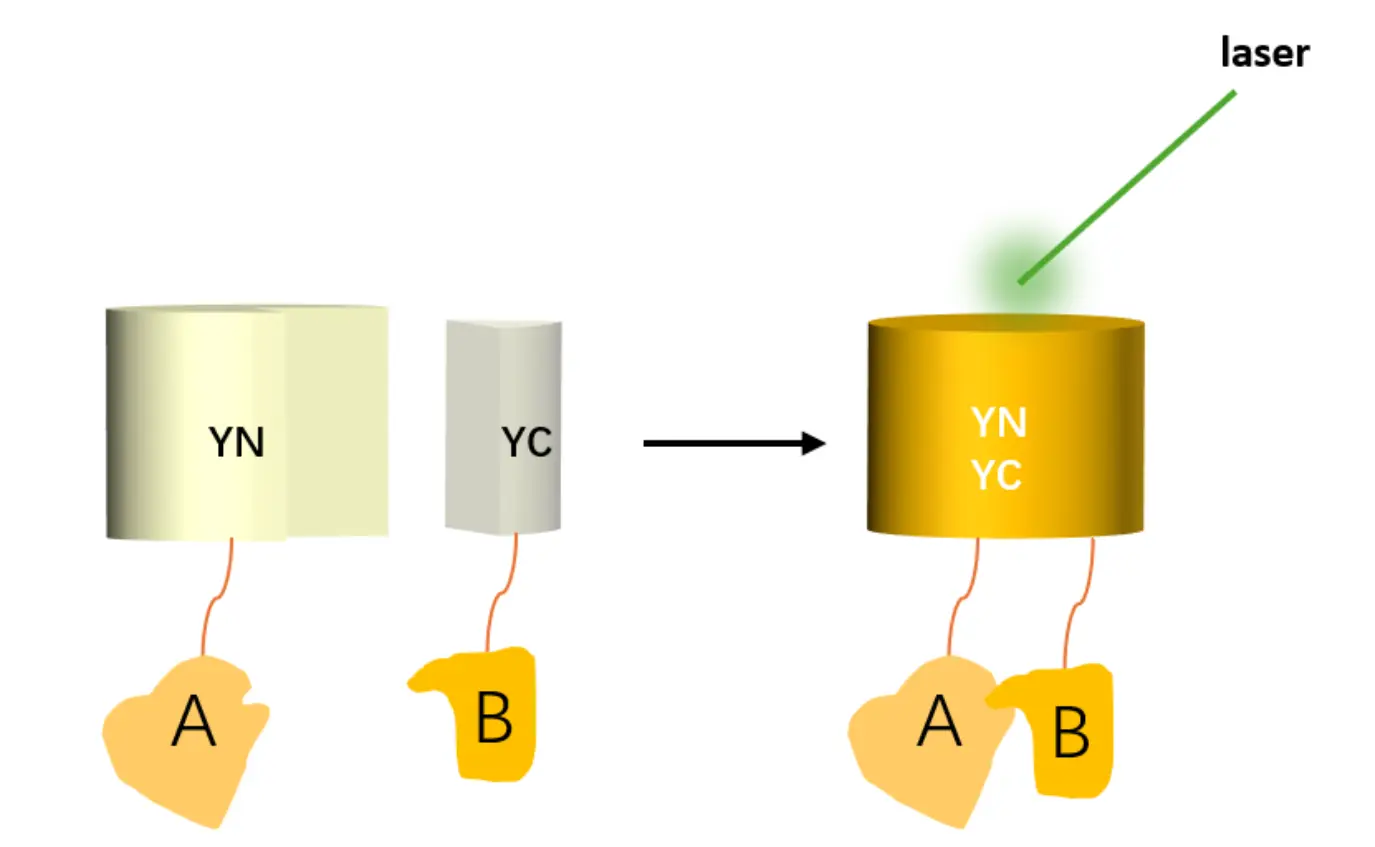
The Bimolecular Fluorescence Complementation (BiFC) assay is a powerful and non-invasive method for detecting protein-protein interactions (PPIs) in living cells. In this technique, a fluorescent protein is split into two non-fluorescent fragments (N-fragment and C-fragment), which are individually fused to two target proteins (A and B). When proteins A and B interact, the fragments come together to reassemble into a functional fluorescent molecule, emitting fluorescence upon excitation. If no interaction occurs, fluorescence will not be observed.
Service Description
The BiFC assay involves the splitting of a fluorescent protein into two inactive fragments: N-fragment and C-fragment. These fragments are fused to two proteins of interest (A and B). Upon interaction between proteins A and B, the two fragments combine to form a functional, fluorescent protein, which emits detectable fluorescence upon excitation. If no interaction is observed, fluorescence will not occur.
This assay is commonly used to verify protein-protein interactions and complement results from other methods such as yeast two-hybrid (Y2H) and co-immunoprecipitation (Co-IP).
Applications
Validation of Protein-Protein Interactions: BiFC is particularly useful for confirming protein interactions that are observed in other assays such as yeast two-hybrid and Co-IP.
In Vivo and In Vitro Studies: Ideal for studying protein interactions both in living cells and in vitro, with the ability to observe cellular localization of interactions.
Key Advantages
Versatility: Suitable for validating both in vivo and in vitro protein interactions.
Natural Protein State: Proteins remain in their natural state, and interaction sites within cells can be visually observed, reducing experimental time.
Simple Equipment & Data Analysis: Unlike other techniques like FRET or BRET, BiFC requires only a fluorescent inverted microscope and straightforward data processing, making it more accessible and cost-effective.
Detection of Weak or Transient Interactions: BiFC is highly effective for studying weak or transient protein-protein interactions that are difficult to capture by other methods.
No Stoichiometric Balancing Required: This assay can detect interactions across various protein subpopulations without the need for precise stoichiometric ratios.
BiFC Assay Workflow
1. Subcellular Localization Prediction
Bioinformatics tools are used to predict potential interaction sites within the cell based on the CDS sequences provided. This helps guide expectations for BiFC signal localization (prediction results are for reference only).
2. Gene Cloning and Vector Construction
CDS sequences of candidate proteins A and B are cloned into pCAMBIA1300-nYFP and pCAMBIA1300-cYFP vectors, respectively.
3. Transformation and Expression
Recombinant plasmids are first transformed into E. coli for amplification.
Plasmids are then transformed into Agrobacterium tumefaciens (GV3101 strain).
Agro-infiltration is performed on young and healthy tobacco (Nicotiana benthamiana) leaves.
4. Fluorescence Detection
After incubation, tobacco leaves are analyzed using confocal laser scanning microscopy to visualize fluorescence signals and determine protein interaction and localization.
Sample Submission Requirements
To initiate the BiFC assay, please provide:
CDS Sequences for each of the two proteins to be tested.
BiFC Service Content and Delivery
Service Content | Working days | Delivery materials |
| 1. Subcellular Localization Prediction | ≤1 | 1. Recombinant Plasmid from Gene Synthesis/Vector Construction and Sequencing Verification Report (Optional); 2. Raw Data and Result Images ( including 1 experimental group, 1 positive control, and 2 negative controls. Each control group includes YFP, Bright field, and Merge images. The experimental group includes 3 visual fields, each with YFP, Bright field, and Merge images); 3. Standard Experimental Report (including experimental procedures and workflows). |
2. Gene Synthesis/Vector Construction:
| 10 | |
3. Tobacco Injection 4. Fluorescence Detection in Tobacco Leaves | 8 | |
5. Reporting and Data Delivery | 2 |
Experimental System
This assay is typically conducted using Nicotiana benthamiana (Tobacco) as the plant model.
Controls: A positive control should fluoresce, while a negative control should not, ensuring assay validation.
Interaction Confirmation: If fluorescence is detected in the experimental group, it confirms interaction between proteins A and B.
Case Study
Using A-nYFP and nYFP-B combinations as negative controls, we confirmed the interaction between proteins A and B in the cell nucleus. This study effectively excluded the vector's influence, ensuring the interaction was specific.
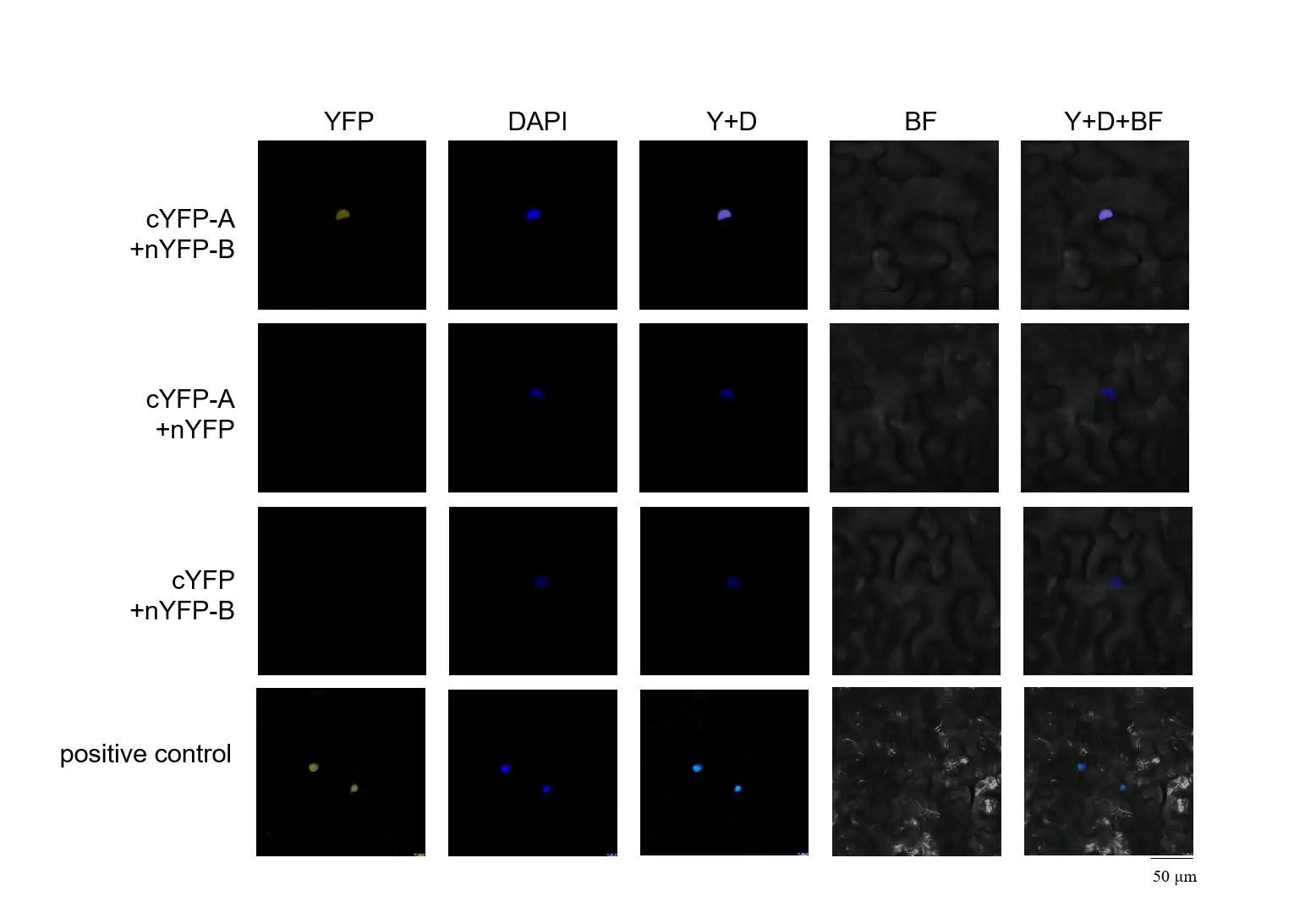
Fig. 1 Interaction of A and B in tobacco
References
Wenyuan Ruan, Meina Guo, Xueqing Wang, et al. “Two RING-Finger Ubiquitin E3 Ligases Regulate the Degradation of SPX4, An Internal Phosphate Sensor, for Phosphate Homeostasis and Signaling in Rice.” Molecular Plant, 2019; 12 (8):1060-1074.
Brett Burdo, John C. Gray, M. Paula Goetting-Minesky, et al. “The Maize TFome – Development of a Transcription Factor Open Reading Frame Collection for Functional Genomics.” The Plant Journal, 2014; 80 (2):356-366.
Bin Ou, Kangquan Yin, Sai-Nan Liu, et al. “A High-Throughput Screening System for Arabidopsis Transcription Factors and Its Application to Med25-Dependent Transcriptional Regulation.” Molecular Plant, 2011; 4 (3):546-555.
BiFC FAQs
1. How can I be sure the fluorescence signal indicates a real protein-protein interaction?
To avoid false positives, appropriate negative controls must be included. Non-specific reassembly of fluorescent fragments or background interactions may lead to misleading signals. We recommend using constructs expressing only one half of the fluorescent protein, or fusions with non-target proteins, to eliminate non-specific complementation.
2. Are there any special experimental conditions required for BiFC assays?
Yes. Temperature plays a critical role in successful BiFC experiments. Since the folding and reassembly of fluorescent protein fragments are temperature-sensitive, it's important to perform experiments under controlled, optimal temperatures (typically 22–25°C) to ensure efficient complementation and reliable signal.
Why Choose Pronetbio for BiFC Assays?
At Pronetbio, we offer high-quality BiFC assays tailored to your research needs. Our advanced platforms and expertise in protein interaction studies make us the trusted partner for researchers around the world. With easy sample submission and comprehensive service offerings, we provide both in vivo and in vitro solutions for your protein interaction studies.
Contact us today to discuss your BiFC assay needs and get started with your project!
Disclaimer: All services offered are for research purposes only and are not intended for diagnostic or therapeutic use.
Services Workflow

Online Consultation
01

Solution Matching
02

Service Contract
03

One-Stop-Services
04

Project Report
05
Related Products
Product Inquiry