Literature Sharing | Molecular mechanism of SmMYB53 activates the expression of SmCYP71D375, thereby modulating tanshinone accumulation in Salvia miltiorrhiza
Release time:
2025-04-25
This study uncovers the transcriptional regulatory network controlling SmCYP71D375, a key enzyme involved in tanshinone biosynthesis in Salvia miltiorrhiza. Using the promoter of SmCYP71D375 as bait in a yeast one-hybrid screen, researchers identified SmMYB53, an R2R3-MYB transcription factor, as an upstream regulator. Overexpression of SmMYB53 in transgenic hairy roots increased SmCYP71D375 expression and promoted tanshinone accumulation, while RNAi-mediated silencing of SmMYB53 reduced it. Further investigation revealed that SmMYB53 physically interacts with SmbZIP51, as confirmed by yeast two-hybrid, GST pull-down, and BiFC assays. This interaction suppresses SmMYB53's activation of SmCYP71D375. Overall, the study defines a regulatory module involving SmMYB53 and SmbZIP51 that modulates tanshinone biosynthesis, providing a foundation for the genetic improvement of medicinal compound production in S. miltiorrhiza.

Due to the vital role of SmCYP71D375 involved in tanshinone biosynthesis and the multiple functions of R2R3-MYB TFs in association with plant secondary metabolism, we employed the two MYB binding sites (MBS1:CAGTTG, MBS2:CCGTTG) in the promoter of SmCYP71D375 to insert the pHIS vector as the bait to conduct yeast one-hybrid (Y1H) library screening. With the MBS1 cis-element as the bait, 20 candidate proteins were captured from the Y1H library (Supplementary Data Table S1), whereas we only preyed 13 candidate proteins using the MBS2 cis-element as the bait . Merging the above two results, we finally anchored an R2R3 transcription factor, namely SmMYB53 (NCBI accession number KF059407.1), based on a previous report. SmMYB53, which is one of the above 33 preys and was validated previously to participate in terpenoid accumulation in Arabidopsis thaliana, was chosen for further study.
We assembled the open reading fragment (ORF) of SmMYB53 based on an established transcriptome database. Then, special SmMYB53 gene primers were introduced to amplify the ORF sequence of SmMYB53, which was subjected to sequencing for final validation. The cDNA sequence of SmMYB53 contained an ORF 1119 bp in length, encoding a total of 372 amino acids with the predicted molecular weight of 39.67 kDa. We constructed a phylogenetic tree of SmMYB53 protein with 125 MYB members in A. thaliana. This result shows that SmMYB53 belongs to the S23 subgroup together with AtMYB1, AtMYB25, and AtMYB109 in A. thaliana (Fig. 1A). In order to clarify the structural characteristics of SmMYB53, we employed multiple protein sequence alignment of SmMYB53 and four typical R2R3-MYB TFs including SlMYB1 (NCBI accession number Solyc09g011780), AtMYB1 (AT3G09230), AtMYB25 (AT2G39880), AtMYB109 (AT3G55730), and OsMYB1 (LOC4324801). The result showed that the N-terminal of SmMYB53 has the highly conserved R2 and R3 domains (Fig. 1B), implying that it belongs to the R2R3 subgroup of MYB TF families.
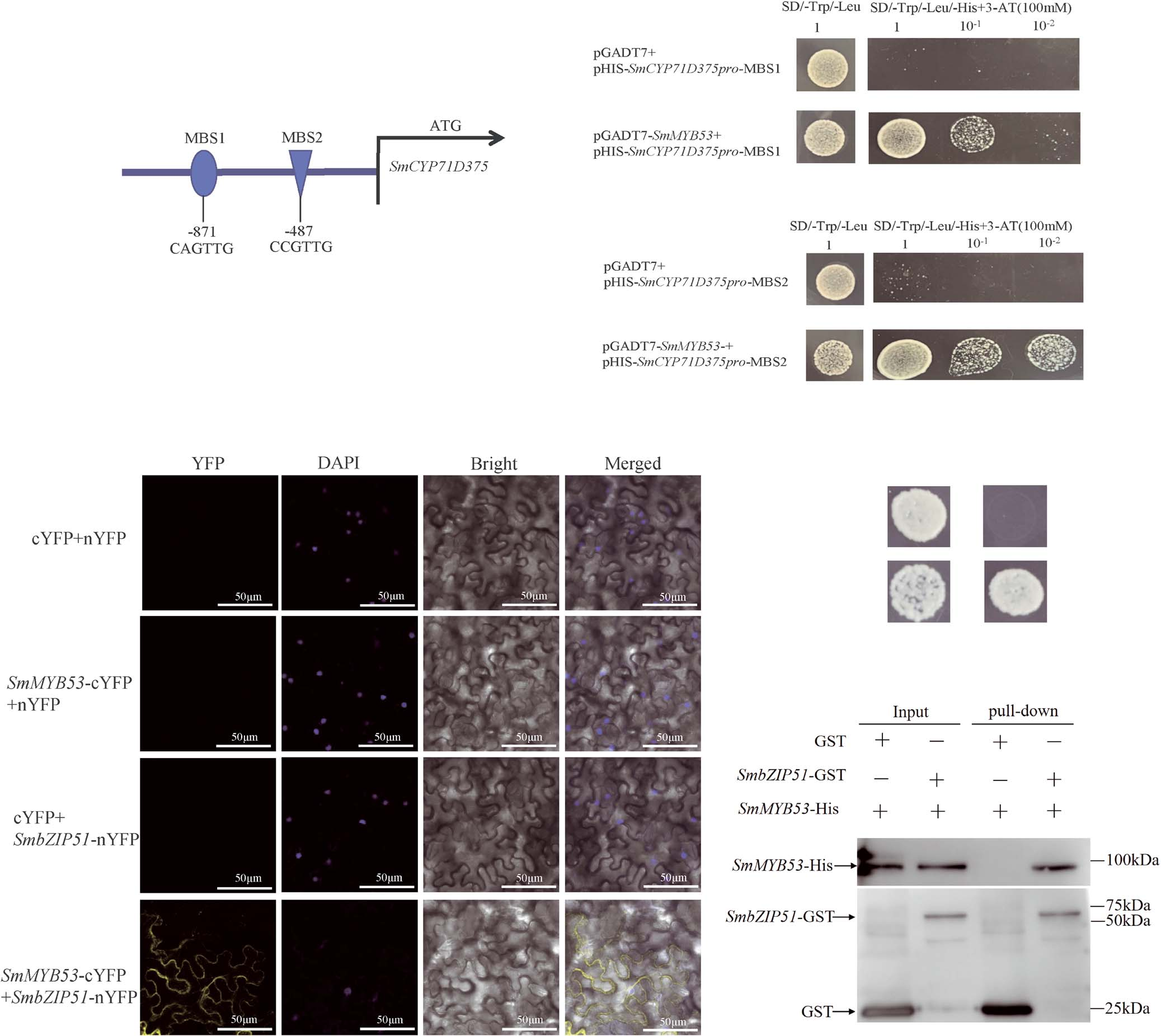
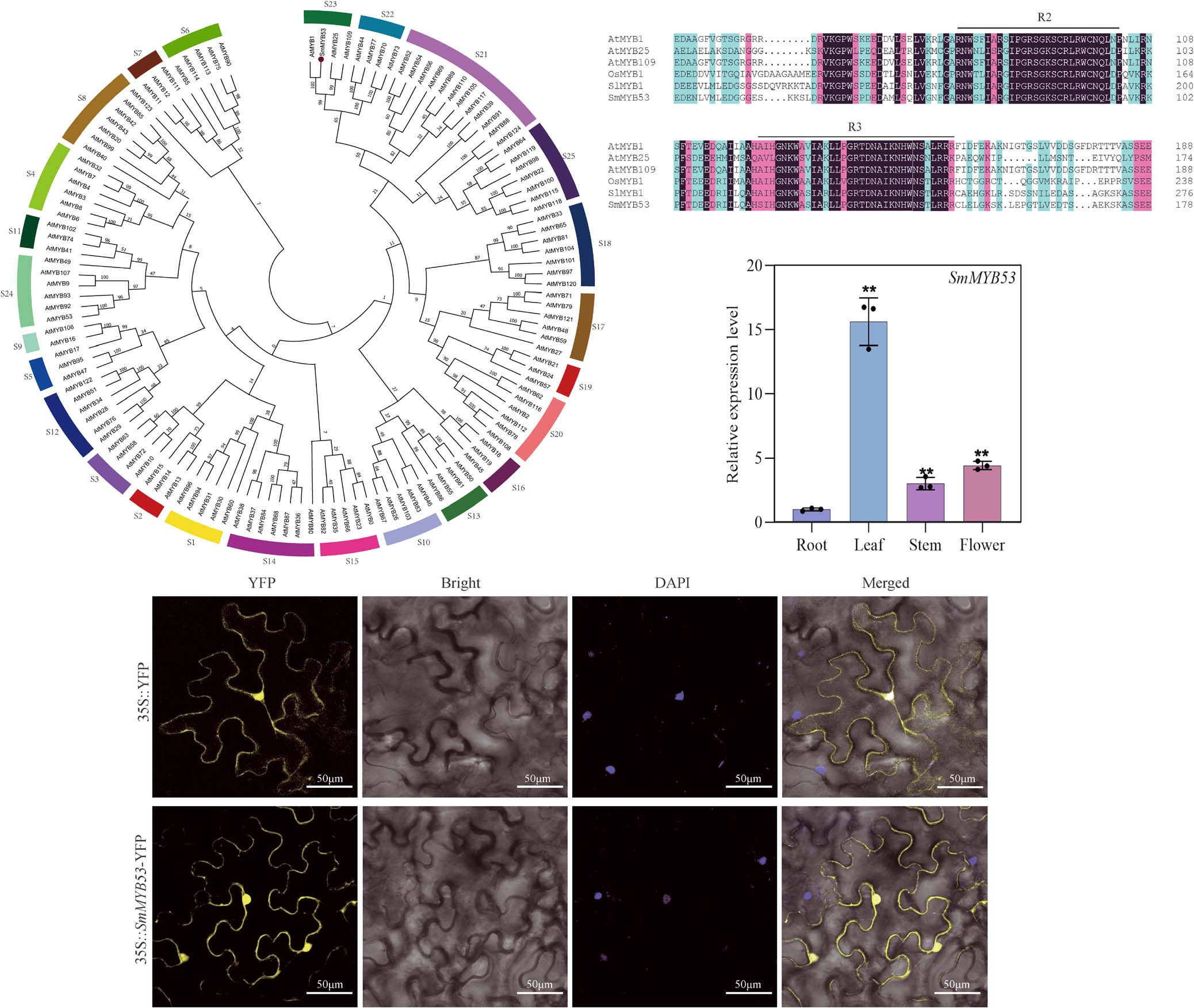
Figure 1. Phylogenetic tree construction, conserved domain, and expression profiles of SmMYB53
We employed qRT–PCR to examine the SmMYB53 expression profile in various tissues, namely, leaf, stem, flower, and root. As shown in Fig. 1C, SmMYB53 was expressed vigorously in the four tissues, and it exhibited the lowest expression level in root and peaked in leaf. Transient expression of SmMYB53 fused with YFP in epidermal cells from Nicotiana benthamiana leaves was used to explore the subcellular localization of SmMYB53. We observed distinct fluorescence in the whole cell with the construct of 35S::SmMYB53-YFP, while the 35S::YFP control also exhibited robust fluorescence signal in the whole cell, implying that SmMYB53 localizes in the whole cell (Fig. 1D).
To investigate the function of SmMYB53 in regulating tanshinone accumulation, transgenic S. miltiorrhiza hairy roots were generated with SmMYB53 overexpression (OE) and RNA interference (RNAi) constructs driven by the CaMV 35S promoter. Transgene integration was confirmed by genomic PCR (Supplementary Data Fig. S3A and B), and lines with the highest (OE-11, -38, -67) and lowest (RNAi-5, -21, -51) SmMYB53 expression were selected for further study (Supplementary Data Fig. S3C and D). HPLC analysis (Fig. 2A and B) revealed significantly increased yields of four tanshinones—TI, TIIA, CT, and DT—as well as total tanshinones (TT) in OE lines (Fig. 2C and D). In particular, SmMYB53-OE-67 accumulated 20 mg/g DW, representing a 3.91-fold increase over the control (Fig. 2E). Conversely, RNAi lines showed dramatically reduced TT levels, with SmMYB53-RNAi-51 dropping to 1.33 mg/g DW (Fig. 2F). Gene expression analysis showed that SmMYB53 upregulated SmCYP71D375, SmKSL1, SmGGPPS1, and SmCYP76AH1 in OE lines (Fig. 2G and Supplementary Data Fig. S4A), but these genes were significantly downregulated in RNAi lines (Fig. 2H and Supplementary Data Fig. S4B). These results demonstrate that SmMYB53 enhances tanshinone biosynthesis by activating multiple key biosynthetic genes.
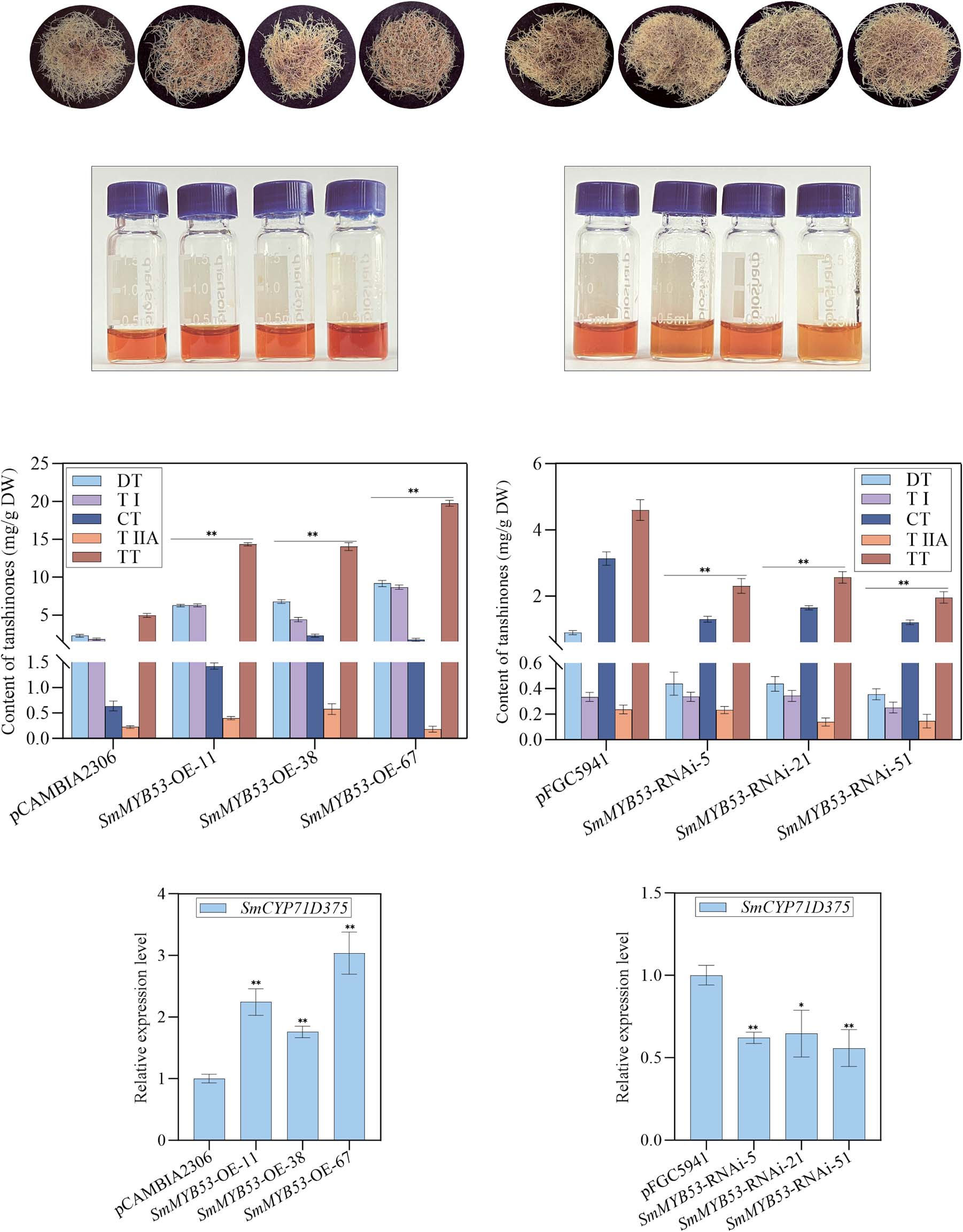
Figure 2. Tanshinone yield and expression profile of SmCYP71D375 in SmMYB53 transgenic hairy roots.
Y1H was used to further validate SmMYB53 binding to the SmCYP71D375 promoter. The Y1H assay showed that SmMYB53 could bind to the MBS1 (CAGTTG) and MBS2 (CCGTTG) cis-elements in the SmCYP71D375 promoter (Fig. 3A-C). Collectively, these results substantiate that SmMYB53 binds to the MBS1 and MBS2 cis-elements within the SmCYP71D375 promoter.
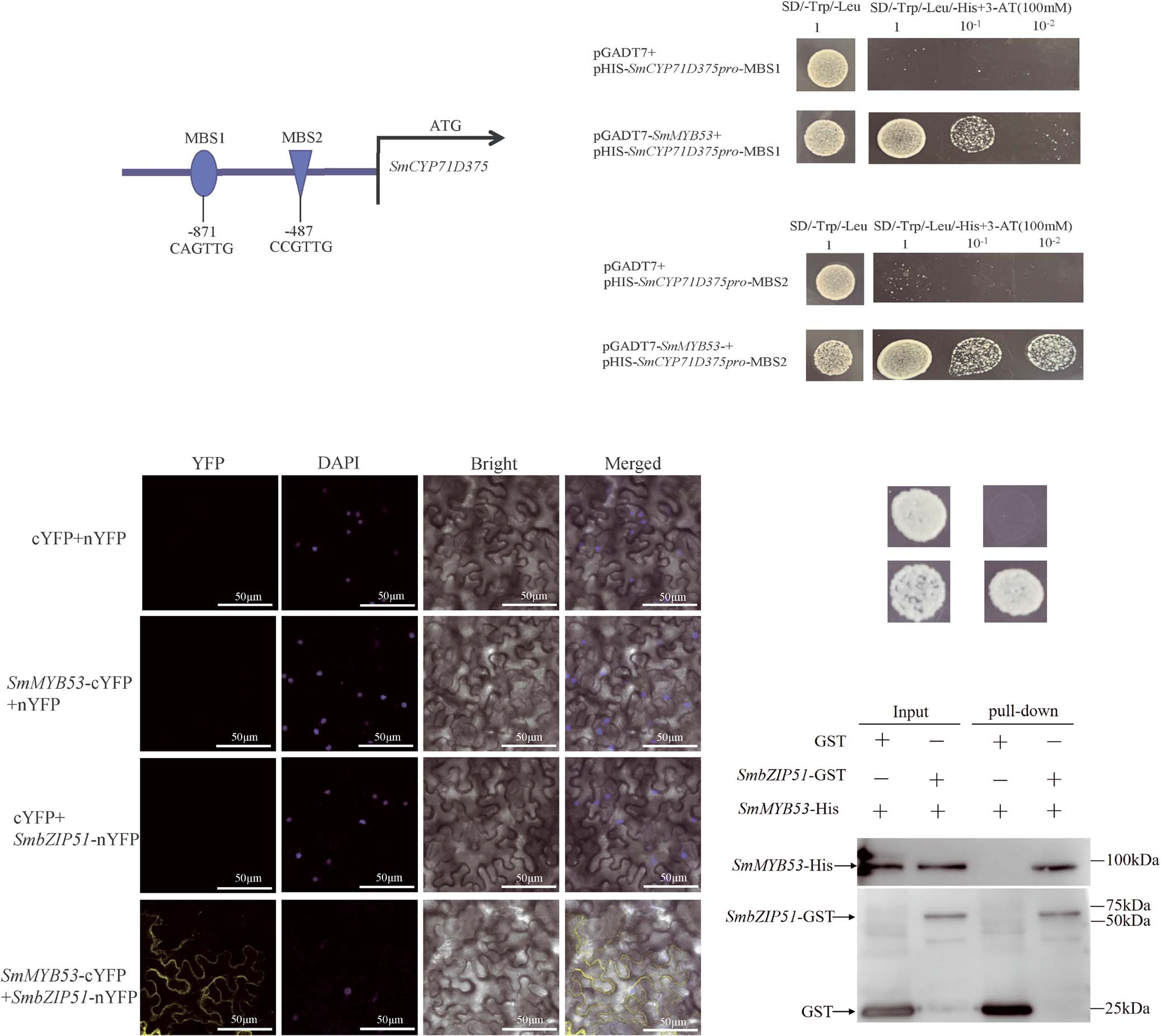
Figure 3. Validation of SmMYB53 binding to the SmCYP71D375 promoter and interaction of SmbZIP51 with SmMYB53.
Related News
2025-04-25
2025-04-22
A Comprehensive Overview – Dual-Luciferase Reporter Gene Assay
2025-04-18
2025-04-15
2025-04-10
A Comprehensive Guide to Transcription Factor Research Strategies (Part II)
2025-04-08
2025-04-01
A Comprehensive Guide to Transcription Factor Research Strategies (Part 1)
2025-03-27